Tetra-, hexa- and octanuclear copper hydride complexes supported by tridentate phosphine ligands†
Abstract
Multinuclear copper hydride complexes were synthesized by using a triphosphine, bis(diphenylphosphinomethyl)phenylphosphine (dpmp). The reaction of [Cu(MeCN)4]PF6 with dpmp in 2 : 1 ratio in the presence of Me4NBH4 or NaBH4 yielded a hexanuclear complex, [Cu6(μ3-H)5(μ-dpmp)3]PF6 (1), together with a minor product [Cu8(μ-H)2(μ4-H)4(μ-dpmp)4](PF6)2 (2) in a very low yield. Complex 1 was also prepared from [CuH(PPh3)]6, [Cu(MeCN)4]PF6, and dpmp in 87%, but the yield of 2 could not be improved presumably due to its instability in solution. A tetranuclear complex, [Cu4(μ-H)(μ3-H)2(μ-dpmp)3]PF6 (4), was obtained from the reaction of [Cu(MeCN)4]PF6 with dpmp in 4 : 3 ratio, or that of [Cu3(μ-dpmp)2(MeCN)4](PF6)3 (3) and dpmp, in the presence of Me4NBH4 for both cases. The structures of 1, 2, and 4 were determined by X-ray crystallography, and the positions of hydride ligands were elucidated by DFT optimization. Complex 1 consists of a distorted trigonal anti-prismatic Cu6 core bridged by three L-shaped dpmp ligands and five μ3-hydrides, and 2 is composed of three edge-shared tetrahedral Cu4 units supported by four L-shaped dpmp ligands and two μ2-hydrides in the two outer Cu4 units and four μ4-hydride ligands in the central Cu4 units. The structures of two outer Cu4 units in 2 resemble those of 4, where a Cu4 tetrahedron is supported by two L-shaped dpmp ligands and one dpmp-κ2P,P′ bridge, in addition to one μ2- and two μ3-hydrides. Natural bond orbital analyses suggest that the hydrides act as glue between the multinuclear copper centres through electron-deficient delocalized bonding interactions, which result in partial electron migration from hydrides to CuI centres.
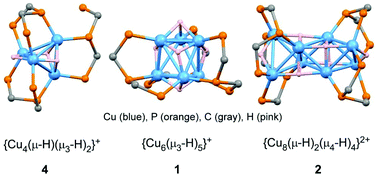
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...