Coordination of a bis-histidine-oligopeptide to Re(i) and Ga(iii) in aqueous solution†
Abstract
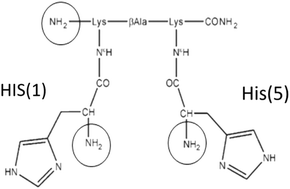
The utilization of isotopes of transition metals for the development of novel therapeutic or diagnostic compounds is limited by the fact that they must be stabilized by chelating systems in coordination complexes. Important roles in the targeting approach are played by the tricarbonyl complexes of Technetium(I) and Rhenium(I) because they can be readily conjugated to biomolecules to form stable probes. Additionally, 67Ga and 68Ga isotopes of gallium are considered an obvious alternative to 99mTc (M. D. Bartholomä, A. S. Louie, J. F. Valliant and J. Zubieta, Chem. Rev., 2010, 110, 2903–2920) for SPECT and PET applications. We have previously reported the characterization of the peptide CCK8 decorated with a bis-histidine-based chelator (pHis2) labeled with 99mTc-tricarbonyl. In order to study the molecular properties of the histidine-based chelator pHis2, we here present the characterization in solution of its complexes with the metals Re(I) and Ga(III) using potentiometry and NMR. We detail the solution equilibria reporting pHis2 acid–base behavior, the coordination properties of pHis2 toward fac-[Re(H2O)3(CO)3]+ and Ga(III) and the atomic details of the formed complexes. Interestingly, two different metal coordination modes were found highlighting the plasticity of this bifunctional chelator.


 Please wait while we load your content...
Please wait while we load your content...