Synthesis and structural characterization of 20-membered macrocyclic rings bearing trans-chelating bis(N-heterocyclic carbene) ligands and the catalytic activity of their palladium(ii) complexes†
Abstract
Macrocycles consisting of a 20-membered ring containing two imidazolium salt functionalities and of the formula [PhCH2N(CH2CH2CH2)Im(CH2CH2CH2)2][Br]2 (Im = imidazole = 3a, benzimidazole = 3b) were synthesized in 70–75% yields. These salts serve as precursors to macrocycles containing two N-heterocyclic carbene (NHC) moieties. Reaction of the macrocyclic salts 3a and 3b with silver oxide afforded macrocyclic-bis(NHC)silver(I) complexes 4a and 4b. Single-crystal X-ray diffraction studies of macrocyclic-bis(NHC)silver(I) complex 4a revealed a tetranuclear silver core with a short Ag–Ag distance (2.9328 Å). Complexes 4a and 4b serve as carbene transfer reagents to Pd. The treatment of macrocyclic-bis(NHC)silver(I) complexes 4a and 4b with one equivalent of PdCl2(MeCN)2 in methylene chloride afforded square-planar trans-macrocyclic-bis(NHC)Pd(II)X2 complexes 5a and 5b. Preliminary screening of these palladium complexes showed they are competent precatalysts for Heck and Suzuki coupling reactions.
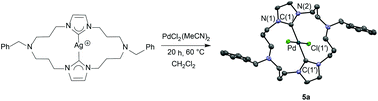


 Please wait while we load your content...
Please wait while we load your content...
