Superior catalytic effects of FeCo nanosheets on MgH2 for hydrogen storage†
Abstract
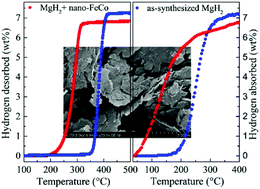
Magnesium hydride (MgH2) is considered as a promising hydrogen storage material for “hydrogen economy” due to its high capacity; however, its stable thermodynamics and slow kinetics hinder its practical applications. Transition metal catalysts attract intense interest in modifying MgH2 systems. Herein, FeCo nanosheets with a thickness of 50 nm were successfully prepared and confirmed to have superior catalytic effects on MgH2. The nano-FeCo-catalyzed MgH2 started to release hydrogen at 200 °C which ended at 320 °C, while the hydrogen desorption process of pure MgH2 occurred at 350–420 °C. Besides, the dehydrogenated FeCo-containing sample could rapidly take up 6.7 wt% H2 within 1 min at 300 °C. Furthermore, after doping with nano-FeCo, the activation energy of hydrogen desorption and absorption was dramatically reduced to 65.3 ± 4.7 kJ mol−1 and 53.4 ± 1.0 kJ mol−1, respectively. In a word, our findings may provide references for designing and producing nano-level intermetallic catalysts for the research area of hydrogen storage or other energy-related research.


 Please wait while we load your content...
Please wait while we load your content...