A copper(ii)-coordination polymer based on a sulfonic–carboxylic ligand exhibits high water-facilitated proton conductivity†
Abstract
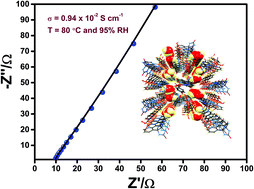
Proton conduction ability has been investigated in a new Cu(II) based coordination polymer (CP), {[Cu2(sba)2(bpg)2(H2O)3]·5H2O}n (1), synthesized using the combination of 4-sulfobenzoic acid (4-Hsba) and bipyridine-glycoluril (BPG) ligands. Single crystal X-ray structure determination revealed that 1 features 1D porous channels encapsulating a continuous array of water molecules. Proton conductivity measurements reveal a high conductivity value of 0.94 × 10−2 S cm−1 at 80 °C and 95% RH. The activation energy (Ea) of 0.64 eV demonstrates that the solvate water, coordinated water and hydrophilic groups in the channels promote the mobility of protons in the framework. Water sorption measurements exhibited hysterical behaviour with a high uptake value of 379.07 cm3 g−1. Time-dependent measurements revealed that the proton conductivity is retained even after 12 h of measurements. The proton conduction mechanism was validated by ab initio electronic structure calculations using the Nudged Elastic Band (NEB) method with molecular dynamics (MD) simulation studies. The theoretical activation energy is calculated to be 0.54 eV which is in accordance with the experimental value.


 Please wait while we load your content...
Please wait while we load your content...