Biological evaluation of optically pure chiral binuclear copper(ii) complexes based on a rosin derivative as highly potential anticancer agents†
Abstract
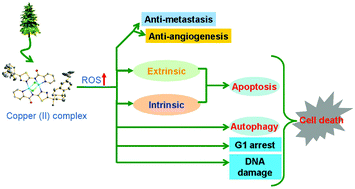
Two optically pure chiral binuclear copper(II) complexes [Cu2(μ-Cl)2L2]·CH2Cl2 (1) and Cu2L4 (2) based on the natural product rosin derivative N-(5-dehydroabietyl-1,3,4-thiadiazole)-2-substituted pyridinecarboxamide (HL) were prepared, fully characterized and their biological activities were evaluated. The circular dichroism (CD), fluorescence spectroscopy, and DNA melting studies indicate that 1 and 2 interact with calf thymus DNA (CT DNA) via intercalation. It can be concluded that 1 and 2 have a strong affinity to bovine serum albumin (BSA) based on the fluorescence and CD spectral evidence. The MTT assay illustrates that the selective cytotoxic activity of 1 is better than that of HL, 2, cisplatin and oxaliplatin. The exposure of 1 to MCF-7 cells resulted in cell cycle arrest in the G1 phase, apoptosis, mitochondrial dysfunction and an elevated ROS level. The western blot analysis results indicate that 1 might induce apoptosis through intrinsic and extrinsic pathways, autophagy and DNA damage in MCF-7 cells. Furthermore, the down-regulated VEGFR2, MMP-2 and MMP-9 expression levels indicate that 1 should have the ability to resist metastasis and angiogenesis. Thus, based on the above described results 1 has high potential value for anticancer applications.
- This article is part of the themed collection: Modern coordination chemistry


 Please wait while we load your content...
Please wait while we load your content...