Phosphaisonitrile umpolung – synthesis and reactivity of chloro aminophosphino carbynes†
Abstract
Sequential treatment of [W(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CBr)(CO)2(Tp*)] (Tp* = hydrotris(dimethylpyrazolyl)borate) with nBuLi and Cl2PNiPr2 provides the aminochlorophosphinocarbyne [W(
CBr)(CO)2(Tp*)] (Tp* = hydrotris(dimethylpyrazolyl)borate) with nBuLi and Cl2PNiPr2 provides the aminochlorophosphinocarbyne [W(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPClNiPr2)(CO)2(Tp*)]. The chloro substituent undergoes subsitution with RLi (R = Me, Et, Ph) to afford [W(
CPClNiPr2)(CO)2(Tp*)]. The chloro substituent undergoes subsitution with RLi (R = Me, Et, Ph) to afford [W(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPRNiPr2)(CO)2(Tp*)] and with [W(
CPRNiPr2)(CO)2(Tp*)] and with [W(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CLi)(CO)2(Tp*)] to give the aminobis(alkylidynyl)phosphine [iPr2NP{C
CLi)(CO)2(Tp*)] to give the aminobis(alkylidynyl)phosphine [iPr2NP{C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) W(CO)2(Tp*)}2]. Treatment with Li[BHEt3] gives the primary aminophosphine [W(
W(CO)2(Tp*)}2]. Treatment with Li[BHEt3] gives the primary aminophosphine [W(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPHNiPr2)(CO)2(Tp*)], formed in a mixture with the ethyl derivative [W(
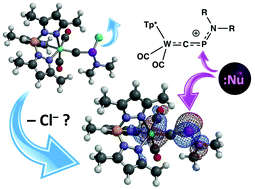
CPHNiPr2)(CO)2(Tp*)], formed in a mixture with the ethyl derivative [W(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPEtNiPr2)(CO)2(Tp*)]. Although they could not be isolated, spectroscopic data indicated that halide abstraction with Al2Cl6 forms the phosphaisonitrile salt [W(CPNiPr2)(CO)2(Tp*)][AlCl4], which displays electrophilic character at phosphorus, e.g., in the reaction with diphenylacetylene to furnish the phosphirenium salt [W{CP(C2Ph2)NiPr2}(CO)2(Tp*)][AlCl4]. Finally, the reaction with LiNPh2 gives the mixed diaminophosphinocarbyne, [W{
CPEtNiPr2)(CO)2(Tp*)]. Although they could not be isolated, spectroscopic data indicated that halide abstraction with Al2Cl6 forms the phosphaisonitrile salt [W(CPNiPr2)(CO)2(Tp*)][AlCl4], which displays electrophilic character at phosphorus, e.g., in the reaction with diphenylacetylene to furnish the phosphirenium salt [W{CP(C2Ph2)NiPr2}(CO)2(Tp*)][AlCl4]. Finally, the reaction with LiNPh2 gives the mixed diaminophosphinocarbyne, [W{![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CP(NiPr2)(NPh2)}(CO)2(Tp*)].
CP(NiPr2)(NPh2)}(CO)2(Tp*)].


 Please wait while we load your content...
Please wait while we load your content...