Aluminium complex as an efficient catalyst for the chemo-selective reduction of amides to amines†
Abstract
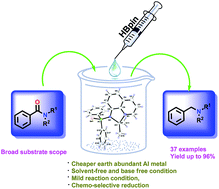
We report an efficient protocol for the catalytic chemo-selective reduction of tert-amides with pinacolborane (HBpin) to afford the corresponding amines in high yields using aluminium complexes [κ2-{Ph2P(X)NC9H6N}Al(Me)2] [X = S (2a), Se (2b)] as pre-catalysts at room temperature. The aluminium complexes were prepared from the reaction of [Ph2P(X)NC9H6N] [X = S (1a), Se (1b)] and trimethylaluminium in toluene. The solid-state structure of complex 2b is established. Tertiary amides with a wide array of electron-withdrawing and electron-donating functional groups were easily converted to the desired products through the selective cleavage of the amides’ C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond by aluminium hydride as an active species. A kinetic study of the catalytic reaction is also reported.
O bond by aluminium hydride as an active species. A kinetic study of the catalytic reaction is also reported.


 Please wait while we load your content...
Please wait while we load your content...