Stepwise oxidations of a nickel(ii)–iron(iii) heterobimetallic porphyrin dimer: structure, spectroscopic and theoretical investigation†
Abstract
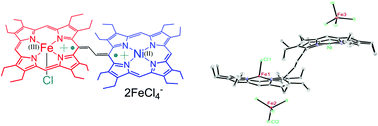
We have reported here the synthesis, structure and spectroscopic properties of the NiII–FeIII heterobimetallic ethene-bridged porphyrin dimer and investigate the effect upon step-wise one- and two-electron oxidations to produce a mixed-valence π–cation radical dimer and a dication diradical complex, respectively. We then investigate their electronic structure and spin coupling model and compare them with the corresponding homobimetallic analog. Detailed UV-vis-NIR, IR, and variable temperature magnetic studies and EPR and NMR spectroscopic investigations along with X-ray structure determination of the 2e-oxidized complex have demonstrated strong electronic communications through the bridge between two porphyrin π–cation radicals. The structure and geometrical parameters revealed the stabilization of the high-spin state of iron and the low-spin state of nickel in the 2e-oxidized complex. The extensive conjugation between the two porphyrin π–cation radicals has also altered the bridge from an ethylene to an exo-methylene moiety. Variable temperature magnetic studies of the 2e-oxidized complex revealed stronger antiferromagnetic coupling between two π–cation radical spins (Jr1–r2) of the NiII–FeIII heterodimer which is in sharp contrast to its diiron(III) analog where the porphyrin π–cation radical undergoes stronger antiferromagnetic coupling predominantly with the corresponding Fe(III) unpaired spin (JFe–r). The experimental observations are also strongly supported by DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...