A study of 99mTc/Re-tricarbonyl complexes of 4-amino-1,8-naphthalimides†
Abstract
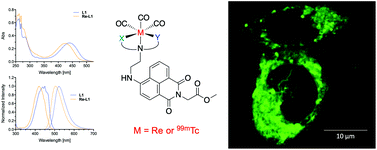
Three 4-amino-1,8-naphthalimide analogues were synthesized, consisting of the tridentate chelators di-2-picolylamine, (pyridin-2-ylmethyl)glycinate, and iminodiacetate conjugated to the naphthalimide scaffold. Coordination with fac-99mTc/Re(CO)3 resulted in metal complexes with overall charges of −1, 0, or +1. Upon coordination of Re(I), the initial naphthalimide-based fluorescence is largely maintained for both negative and neutral complexes compared to their free ligand forms, while an increase in fluorescence quantum yield was observed for the positively charged complex. OVCAR-8 ovarian cancer cells were stained with each of the complexes, demonstrating that the positive complex is more lipophilic and cell membrane permeable than the neutral and negative complexes. Each of the three technetium-99m labelled naphthalimide complexes were successfully produced from fac-[99mTc(CO)3(H2O)3]+ in 15 minutes at 70 °C and isolated in radiochemical yields ranging from 60–95% with radiochemical purities greater than 95%. These fluorescent metal complexes of various charges can be used to tune pharmacokinetic and cellular uptake properties of 99mTc/Re-naphthalimide-bioconjugates, while still maintaining desirable fluorescence properties.


 Please wait while we load your content...
Please wait while we load your content...