Synthesis, characterisation and water–gas shift activity of nano-particulate mixed-metal (Al, Ti) cobalt oxides†
Abstract
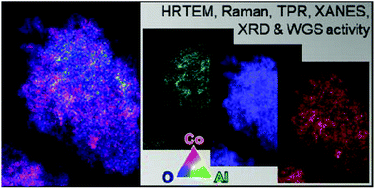
The formation of mixed-metal cobalt oxides, representing potential metal–support compounds for cobalt-based catalysts, has been observed at high conversion levels in the Fischer–Tropsch synthesis over metal oxide-supported cobalt catalysts. An often observed increase in the carbon dioxide selectivity at Fischer–Tropsch conversion levels above 80% has been suggested to be associated to the formation of water–gas shift active oxidic cobalt species. Mixed-metal cobalt oxides, namely cobalt aluminate and cobalt titanate, were therefore synthesised and tested for potential catalytic activity in the water–gas shift reaction. We present a preparation route for amorphous mixed-metal oxides via thermal treatment of metal precursors in benzyl alcohol. Calcination of the as prepared nanoparticles results in highly crystalline phases. The nano-particulate mixed-metal cobalt oxides were thoroughly analysed by means of X-ray diffraction, Raman spectroscopy, temperature-programmed reduction, X-ray absorption near edge structure spectroscopy, extended X-ray absorption fine structure, and high-resolution scanning transmission electron microscopy. This complementary characterisation of the synthesised materials allows for a distinct identification of the phases and their properties. The cobalt aluminate prepared has a cobalt-rich composition (Co1+xAl2−xO4) with a homogeneous atomic distribution throughout the nano-particulate structures, while the perovskite-type cobalt titanate (CoTiO3) features cobalt-lean smaller particles associated with larger ones with an increased concentration of cobalt. The cobalt aluminate prepared showed no water–gas shift activity in the medium-shift temperature range, while the cobalt titanate sample catalysed the conversion of water and carbon monoxide to hydrogen and carbon dioxide after an extended activation period. However, this perovskite underwent vast restructuring forming metallic cobalt, a known catalyst for the water–gas shift reaction at temperatures exceeding typical conditions for the cobalt-based Fischer–Tropsch synthesis, and anatase-TiO2. The partial reduction of the mixed-metal oxide and segregation was identified by means of post-run characterisation using X-ray diffraction, Raman spectroscopy, and transmission electron microscopy energy-dispersive spectrometry.


 Please wait while we load your content...
Please wait while we load your content...