Formation of an NIR-emitting Ag34S3SBB20(CF3COO)62+ cluster from a hydride-protected silver cluster†
Abstract
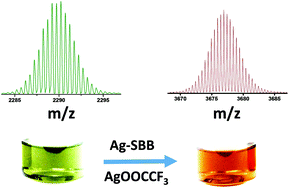
Recent reports have shown that the intercluster reaction is a new synthetic strategy to prepare alloy clusters. In this work, we performed an intercluster reaction between silver clusters and produced highly ionizable Ag–S-type clusters; we examined their formation by mass spectrometry. [Ag18(TPP)10H16]2+ (Ag18), a highly reactive hydride and phosphine-protected silver cluster, was used as a sacrificial cluster in this synthesis. An intercluster reaction between Ag18 and smaller silver-chalcogenolate clusters (SCC) resulted in a new cluster, [Ag34S3SBB20(CF3COO)6]2+. The cluster showed an NIR emission at around 1100 nm. The cluster composition was confirmed by high-resolution electrospray ionization mass spectrometry (ESI-MS), thermogravimetry (TGA), and X-ray photoelectron spectroscopy (XPS).


 Please wait while we load your content...
Please wait while we load your content...