Electrocatalytic reduction of dioxygen by Mn(iii) meso-tetra(N-methylpyridinium-4-yl)porphyrin in universal buffer†
Abstract
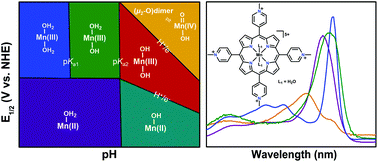
The electrochemical characterization of manganese(III) meso-tetra(N-methylpyridinium-4-yl)porphyrin pentachloride ([Mn(TMPyP)Cl][Cl]4) via cyclic voltammetry (CV) and UV-vis spectroelectrochemistry (UV-vis SEC) was performed across the entire pH domain in aqueous buffered conditions. Assessment of the homogeneous electrocatalytic efficiency for the oxygen reduction reaction (ORR) from pH 3 to 6 using rotating-ring disk electrode experiments (RRDE) found it to be selective for water (82 to 93%). The observed efficiency for water is in contrast to previous reports on electrocatalytic ORR activity by Mn porphyrins in aqueous systems, which identified H2O2 as the primary product using indirect RDE methods only. The results described here are consistent with recent reports on the electrocatalytic behavior of Mn porphyrins under nonaqueous conditions, where the similar selectivity for water was also determined by RRDE methods. At pH 1, UV-vis SEC experiments also revealed that decomposition was occurring; free-base porphyrin was observed after the application of reducing potentials.


 Please wait while we load your content...
Please wait while we load your content...