Bis(trimethylsilyl)amide complexes of s-block metals with bidentate ether and amine ligands†
Abstract
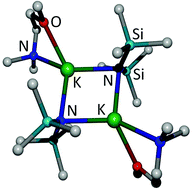
The synthesis of the bistrimethylsilylamide complexes of alkali and alkaline-earth metals with bidentate ether and amine bases 1,2-bis(dimethylamino)ethane (tetramethylethylenediamine, tmeda), dimethyl-methoxyethylamine (dmmea), and 1,2-dimethoxyethane (dme) succeeds via addition of these bases to coligand-free complexes or via ligand exchange of thf adducts. The lithium and sodium complexes are mononuclear (exceptions: [(dme)LiN(SiMe3)2]2 and [(tmeda)NaN(SiMe3)2]2) whereas the heavier congeners form dinuclear compounds with central four-membered M2N2 rings. The alkaline-earth metal complexes crystallize as mononuclear complexes i.e. as [(L)M{N(SiMe3)2}2] with four-coordinate metal centers or as [(L)2M{N(SiMe3)2}2] with six-coordinate metal atoms. Intramolecular steric repulsion leads to distortions such as an asymmetric coordination of L and/or significantly different proximal and distal M–N–Si bond angles.


 Please wait while we load your content...
Please wait while we load your content...