7-Metalla-1,4-diphosphanorbornadienes: cycloaddition of monovalent group 13 NacNac complexes to a stable 1,4-diphosphinine†
Abstract
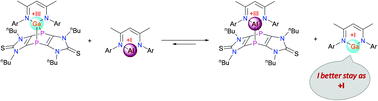
This work presents the first example of a dienophilic property of the monovalent group-13 NacNac complexes, which are well-known for their carbene-like reactivity, e.g., addition to multiple bonds, insertion into single bonds, and oxidative cleavage of some multiple bonds. In reactions with imidazole-2-thione based tricyclic 1,4-diphospinine 4, the monovalent compounds NacNacM (M = Al,Ga) showed dienophilic behaviour and produced the corresponding 7-metalla-1,4-diphosphanorbornadienes (5–6), leaving the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S functionality of the imidazole-2-thione intact, while the NacNacIn complex did not show any reactivity because of unfavourable thermodynamics. DFT calculations revealed that for NacNacAl, the cycloaddition was kinetically more favoured (due to a small reaction barrier) than the oxidative cleavage of the C
S functionality of the imidazole-2-thione intact, while the NacNacIn complex did not show any reactivity because of unfavourable thermodynamics. DFT calculations revealed that for NacNacAl, the cycloaddition was kinetically more favoured (due to a small reaction barrier) than the oxidative cleavage of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S functionality, despite the fact that the product of the C
S functionality, despite the fact that the product of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond addition has a somewhat higher stability. For NacNacGa, the cycloaddition reaction was both kinetically and thermodynamically favourable than the cleavage of C
S bond addition has a somewhat higher stability. For NacNacGa, the cycloaddition reaction was both kinetically and thermodynamically favourable than the cleavage of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S. Furthermore, these reactions were found to be reversible in nature and diphosphinine 4 showed a clear preference towards Al than towards Ga which reflects the better inclination of Ga to stay monovalent than be in the trivalent state.
S. Furthermore, these reactions were found to be reversible in nature and diphosphinine 4 showed a clear preference towards Al than towards Ga which reflects the better inclination of Ga to stay monovalent than be in the trivalent state.


 Please wait while we load your content...
Please wait while we load your content...