Chelate rings of different sizes with non-innocent ligands
Abstract
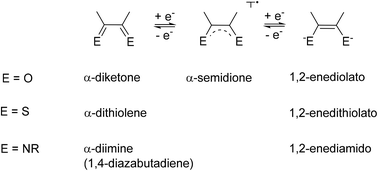
Redox-active unsaturated chelate ligands can be realised with different ring sizes of the resulting metallacycles. An overview is presented, starting from an exposition of non-innocent behaviour and chelate effects. A systematic approach is used to describe the most familiar situation, the metal complexes of 1,4-hetero-1,3-dienes in established forms (e.g. o-quinone, α-dithiolene, and α-diimine ligands) and with less common combinations of O, S, and N heteroatoms. The different steric and electronic conditions in six-membered chelate ring systems derived from the β-diketonate structure will be discussed with examples of substituted and π extended ligands, including 9-oxidophenalenyl, formazanate, and anions derived from indigo or 9,10-anthraquinone. Four-membered chelate rings existing in at least two ligand-based oxidation states are available through steric and electronic stabilisation in amidinate or triazenide complexes. Three-membered and seven-membered chelate ring situations are discussed briefly as further alternatives.
- This article is part of the themed collections: 2019 Frontier and Perspective articles and Nitrogen Ligands


 Please wait while we load your content...
Please wait while we load your content...