Structural and magnetic characterization of Ni(ii), Co(ii), and Fe(ii) binuclear complexes on a bis(pyridyl-triazolyl)alkane basis†
Abstract
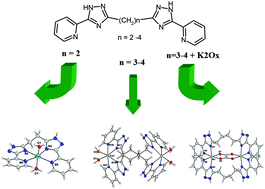
Reactions of bis[5-(2-pyridyl)-1,2,4-triazol-3-yl]alkanes (alkane spacers = –CH2– in L2, –C3H6– in L3, –C4H8– in L4) with M(II)A2 salts (M = Ni, Co, Fe) resulted in the preparation of five series of mononuclear ([M(L2)(H2O)2]2+, 1a–c) or binuclear ([M2(L3)2(H2O)4]4+, 2a–c; ([M2(L4)2(H2O)4]4+, 3a–c, [M2(L3)2(μ-ox)]2+, 4a–c; [M2(L4)2(μ-ox)]2+, 5a–c) complexes. The crystal structures of ten complexes were determined by single-crystal X-ray crystallography. Magnetic properties of the compounds were characterized by SQUID magnetometry and were analyzed by fitting on a spin Hamiltonian model. It was revealed that Fe(II) and Co(II) compounds exhibit non-negligible anisotropy and in the case of 2a–c and 3a–c complexes weak ferromagnetic interactions between the metal centers were observed. In the case of complexes containing an {M2(μ-ox)}2+ core strong antiferromagnetic interactions were observed within the dimer. Remarkably, solid state luminescence of Co(II) and Fe(II) complexes (1b, 2b, 3b and 1c, 2c, 3c) was observed.
- This article is part of the themed collection: Nitrogen Ligands


 Please wait while we load your content...
Please wait while we load your content...