Phosphonium–stibonium and bis-stibonium cations as pnictogen-bonding catalysts for the transfer hydrogenation of quinolines†
Abstract
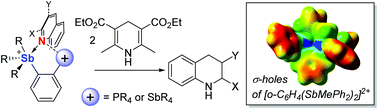
Bifunctional Lewis acidic group 15 compounds have emerged as appealing platforms for anion sensing and organocatalysis. As part of our interest in the chemistry of these compounds, we have now compared the catalytic properties of [o-(MePPh2)C6H4SbPh2]+ ([3]+), [o-(PPh2)C6H4SbPh3]+ ([4]+), [o-(MePPh2)C6H4SbPh3]2+ ([5]2+), and [o-C6H4(SbMePh2)2]2+ ([6]2+) using the transfer hydrogenation of 2-phenyl-quinoline and 3-bromoquinoline with a Hantzsch ester benchmark reactions. This study, which also involved an evaluation of the catalytic properties of [Ph4Sb]+ and [Ph3MeP]+, shows that antimony cations are generally more active than their phosphorus counterparts and neutral stiboranes. Our results also demonstrate that dicationic systems such as [6]2+ are superior activators.


 Please wait while we load your content...
Please wait while we load your content...