New heterobimetallic ferrocenyl derivatives are promising antitrypanosomal agents†
Abstract
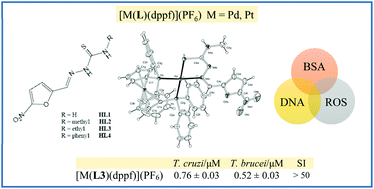
In the search for a more effective chemotherapy for the treatment of Chagas’ disease and human African trypanosomiasis, caused by Trypanosoma cruzi and Trypanosoma brucei parasites, respectively, the use of organometallic compounds may be a promising strategy. In this work, eight new heterobimetallic compounds are described including four 5-nitrofuryl containing thiosemicarbazones as bioactive ligands (HL1–HL4) and dppf = 1,1′-bis(diphenylphosphino) ferrocene as an organometallic co-ligand. Complexes of the formula [MII(L)(dppf)](PF6) with M = Pd or Pt were synthesized and fully characterized in the solid state and in solution, including the determination of the molecular structure of four of them by single crystal X-ray diffraction methods. Most compounds showed activity in the low micromolar or submicromolar range against both parasites, with the platinum compounds being more active than the palladium analogues. Activity was significantly increased by generation of the M-dppf compounds (3–24 fold increase with respect to free ligands HL for T. cruzi and up to 99 fold increase with respect to HL for T. brucei). The inclusion of the organometallic co-ligand also led to lower toxicity in mammalian cells and higher selectivity towards both parasites when compared to the free HL compounds. The complexes interact with DNA and affect the redox metabolism of the parasites. Furthermore, the most active and selective compound of the new series showed no in vivo toxicity in zebrafish embryos.


 Please wait while we load your content...
Please wait while we load your content...