Preparation of CeSiO4 from aqueous precursors under soft hydrothermal conditions†
Abstract
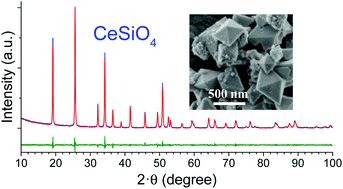
Even though CeSiO4 was synthesized one time through a hydrothermal treatment, the conditions leading to its formation remain largely unknown. In order to define the optimized conditions of synthesis, a multiparametric study was developed by varying the pH of the solution, the temperature, and the nature of the reactants and of the complexing ions in solution. This study highlighted that CeSiO4 could not be obtained starting from Ce(IV) reactants. An optimal set of conditions was defined to prepare single phase samples. Pure CeSiO4 was obtained through a hydrothermal treatment at 150 °C using a starting mixture of 1 mol L−1 Ce(III) nitrate and Na2SiO3 solutions and by adjusting the initial pH to 8. The chemical limitations observed during the synthesis of CeSiO4 suggested that the formation of this phase may result from the slow in situ oxidation of a Ce(III) silicate complex during the hydrothermal treatment.


 Please wait while we load your content...
Please wait while we load your content...