Nucleophilic substitution: a facile strategy for selective B–H functionalization of carboranes
Abstract
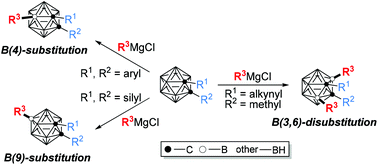
Vertex-specific functionalization of carboranes has received considerable research interest, due to the valuable application of carborane derivatives in medicine, coordination/organometallic chemistry and materials. In comparison with a protic cage C–H bond, cage B–H is hydridic and generally less polar, with a bond dissociation energy of ca. 108 kcal mol−1. These features make B–H activation quite different from that of the C–H bond. In addition, selectivity among ten very similar BH vertices in o-carborane is challenging yet crucial to the effective construction of carborane-based functional molecules. To address these issues, a brand new strategy of cage B–H nucleophilic substitution has recently been presented and developed for straightforward and regioselective cage B–H functionalization. The regioselectivity can be controlled by the electronic/steric properties of the cage carbon substituents. This Frontier article highlights the recent advancement in the nucleophilic cage B–H substitution of carboranes.
- This article is part of the themed collection: 2019 Frontier and Perspective articles


 Please wait while we load your content...
Please wait while we load your content...