Synthesis of a hydrogen-bridged tetraborane(6): a substituent effect of a diaminoboryl group toward the B–B multiple bond character†
Abstract
A hydrogen-bridging tetraborane(6) was synthesized from boryllithium, a boron nucleophile, in three steps. The structural and spectroscopic analyses of the tetraborane(6) revealed σ-donor and π-acceptor effects of diaminoboryl substituents toward the central B–B moiety having a multiple bond character. DFT calculations also supported the experimentally observed substituent effect of the boryl group.
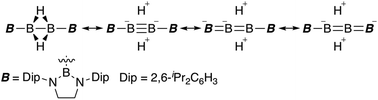
- This article is part of the themed collection: Inorganic chemistry of the p-block elements


 Please wait while we load your content...
Please wait while we load your content...