The sulfonate group as a ligand: a fine balance between hydrogen bonding and metal ion coordination in uranyl ion complexes†
Abstract
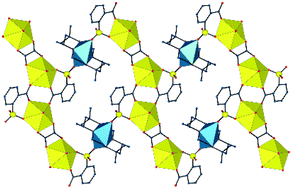
Nine uranyl ion complexes have been synthesized using two kinds of sulfonate-containing ligands, i.e. 2-, 3- and 4-sulfobenzoic acids (2-, 3- and 4-SBH2), which include additional carboxylic donors, and p-sulfonatocalix[4]arene (H8C4S), with additional phenolic groups, and [Ni(cyclam)]2+, [Cu(R,S-Me6cyclam)]2+ or PPh4+ as counterions. [Ni(cyclam)][UO2(4-SB)2(H2O)2]·2CH3CN (1) and [Ni(cyclam)][UO2(3-SB)2(H2O)2] (2) are molecular species in which only the carboxylate groups are coordinated to uranyl, the sulfonate groups being essentially hydrogen bond acceptors. In contrast, uranyl κ1-O(S);κ1-O(C)-chelation is found in the four complexes involving 2-SB2−, different bridging interactions producing diverse geometries. [UO2(2-SB)2Ni(cyclam)]·H2O (3) crystallizes as a two-dimensional (2D) assembly with fes topology, in which uranyl ion dimeric subunits are bridged by six-coordinate NiII cations. Complexes [UO2(2-SB)2Cu(R,S-Me6cyclam)]2·2H2O (4) and [(UO2)2(2-SB)2(C2O4)Cu(R,S-Me6cyclam)] (5), obtained together from the same solution, are a molecular tetranuclear complex and a 2D species with fes topology, respectively, depending on the coordination number, 5 or 6, of the CuII cation. The complex [PPh4]2[(UO2)2(2-SB)3(H2O)]·H2O (6) is a one-dimensional (1D), ribbon-like coordination polymer with a layered packing of alternate cationic and anionic sheets. No heterometallic complex was obtained with H8C4S, but the copper-only compound [{Cu(R,S-Me6cyclam)}5(H3C4S)2]·17H2O (7) displays mixed coordination/hydrogen bonding association of the copper azamacrocycle complex with the phenolic groups. The complexes [PPh4]5[UO2(H4C4S)(H2O)4][UO2(H3C4S)(H2O)4]·14H2O (8) and [PPh4]3[UO2(H3C4S)(H2O)3]·9H2O (9) were crystallized from the same solution and are a molecular complex and a 1D polymer, respectively, with monodentate sulfonate coordination to uranyl, while [PPh4]2[UO2(H4C4S)(H2O)3]·11H2O (10) is also a 1D polymer. The anionic complexes in the last three complexes form layers (9) or double layers (8 and 10) separated from one another by hydrophobic layers of PPh4+ cations. The balance between coordination and hydrogen bonding interactions with the macrocyclic ligands provides an indication of the energy of the sulfonate coordinate bond. Complex 6 is the only luminescent species in this series, albeit with a low quantum yield of 3%, and its emission spectrum is typical of a uranyl complex with five equatorial donors.


 Please wait while we load your content...
Please wait while we load your content...