Phosphine-promoted ring-opening of benzisothiazolinate ligands at a nickel(ii) centre: a convenient synthesis of Ni(ii)-thiolate complexes†
Abstract
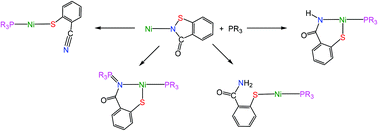
Phosphines react with the benzisothiazolinate (bit) paddlewheel dimer, [Ni2(μ-bit)4·2H2O], resulting in sulfur–nitrogen bond scission and a series of unexpected transformations leading to novel Ni(II) complexes containing 2-cyanophenylthiolate and related thiolate ligands.
- This article is part of the themed collection: Challenges in organometallic & coordination chemistry: in celebration of Geoff Cloke’s 65th birthday


 Please wait while we load your content...
Please wait while we load your content...