Palladium pincer complexes featuring an unsymmetrical SCN indene-based ligand with a hemilabile pyridine sidearm†
Abstract
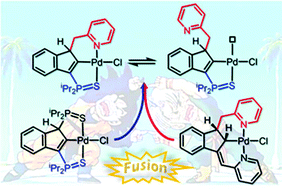
A new unsymmetrical indene-based pro-ligand featuring thiophosphinoyle and methylpyridine sidearms 2 was prepared. Coordination and cyclometalation in the presence of [PdCl2(PhCN)2] and PS-DIEA afforded three well-defined 2-indenyl SCN pincer complexes 3a–c. The lability of the pyridine moiety has been evidenced upon treatment with triphenylphosphine and 2,6-dimethylphenylisocyanide. In addition, reversible C–Pd bond cleavage has been demonstrated under Brønsted acid/base conditions. The indenediide SCN pincer complex 4 was prepared by deprotonation of 3a in the presence of triphenylphosphine. Preliminary catalytic tests on the cycloisomerization of 4-pentynoic acid have underlined the impact of the pyridine sidearm on the catalytic activity.
- This article is part of the themed collection: The central role of the d-block metals in the periodic table


 Please wait while we load your content...
Please wait while we load your content...