Ring size effects in cyclic fluorophosphites: ligands that span the bonding space between phosphites and PF3†
Abstract
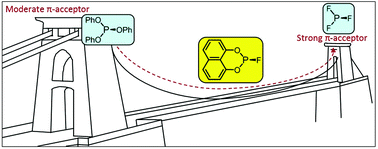
The 5- to 8-membered cyclic fluorophosphites L5–8 have been prepared from the corresponding chlorophosphites which are derived from dihydroxyarenes or bis(trimethylsiloxy)arenes. Ligand L5 is very sensitive to hydrolysis but L6–8 are much more kinetically robust. The coordination chemistry of L5–8 has been explored with Mo(0), Pt(0) and Rh(I) and it is shown that the π-acceptor properties of L5–8 increase with decreasing ring size. The IR spectra and X-ray crystal structures of the [Mo(CO)4L2] complexes show that L5–8 lie between PF3 and P(OAr)3 in terms of their σ/π-bonding properties. The [PtL4] complexes are readily prepared from [Pt(nbe)3] and 4 equiv. of L5–8 whereas equilibrium mixtures of PtLx(nbe)y species form when 2 equiv. of L5–8 are added to [Pt(nbe)3]. The CO substitution reactions of [Rh2Cl2(CO)4] with L5–8 to give [Rh2Cl2L4] are evidence of the PF3-like ligand properties of L5–8. The trends in the properties of L5–8 are analysed in terms of their proximity to PF3 or P(OPh)3.
- This article is part of the themed collection: The central role of the d-block metals in the periodic table


 Please wait while we load your content...
Please wait while we load your content...