Voltammetric characterisation of diferrocenylborinic acid in organic solution and in aqueous media when immobilised into a titanate nanosheet film†
Abstract
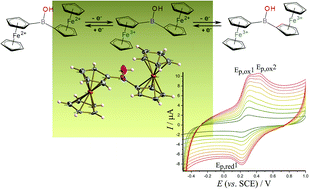
Diferrocenylborinic acid (Fc2BOH, 1) has been synthesized in good yield via an improved synthetic path. Characterisation by nuclear magnetic resonance (NMR), mass spectrometry (HRMS), infrared spectroscopy (FTIR), X-ray crystallography, and by electrochemical methods reveal two one-electron oxidation processes for the two electronically coupled ferrocenyl moieties. The oxidation of 1 dissolved in organic media is contrasted to the oxidation of 1 in aqueous environments (by incorporation of 1 into a lamellar film of 2D titanate nanosheets on a glassy carbon electrode). Data from cyclic voltammetry and from square wave voltammetry suggest that the bridging boron can bind to nucleophiles (hydroxide, fluoride) upon oxidation of the ferrocenyl groups. A multi-pathway ECE reaction scheme is proposed. Potential applications in sensing are discussed.
- This article is part of the themed collection: The Mechanics of Supramolecular Chemistry


 Please wait while we load your content...
Please wait while we load your content...