Deprotonation, insertion and isomerisation in the post-functionalisation of tris-pyridyl aluminates†
Abstract
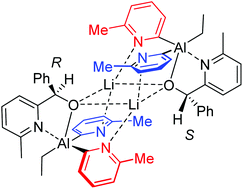
Post-functionalisation of the aluminate anion [EtAl(6-R-2-py)3]− (6-R-2-py = 6-R-2-pyridyl, R = Me or Br) can be accomplished via nucleophilic addition of the pyridyl groups to the electrophilic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of aldehydes (RCH
O group of aldehydes (RCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) or by deprotonation of carboxylic acids (RCO2H). NMR spectroscopic and crystallographic studies show how 6-Me-2-py groups can detect chirality and reveal a new aspect of isomerism.
O) or by deprotonation of carboxylic acids (RCO2H). NMR spectroscopic and crystallographic studies show how 6-Me-2-py groups can detect chirality and reveal a new aspect of isomerism.
- This article is part of the themed collection: Inorganic chemistry of the p-block elements


 Please wait while we load your content...
Please wait while we load your content...