Aluminium(iii) dialkyl 2,6-bisimino-4R-dihydropyridinates(−1): selective synthesis, structure and controlled dimerization†
Abstract
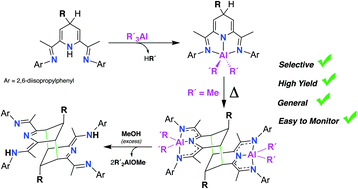
A family of stable and otherwise selectively unachievable 2,6-bisimino-4-R-1,4-dihydropyridinate aluminium (III) dialkyl complexes [AlR'2(4-R-iPrBIPH)] (R = Bn, Allyl; R′ = Me, Et, iBu) have been synthesized, taking advantage of a method for the preparation of the corresponding 4-R-1,4-dihydropiridine precursors developed in our group. All the dihydropyrdinate(−1) dialkyl aluminium complexes have been fully characterized by 1H- 13C-NMR, elemental analysis and in the case 2′a, also by X-ray diffraction studies. Upon heating in toluene solution at 110 °C, the dimethyl derivatives 2a and 2′a dimerize selectively through a double cycloaddition. This reaction leads to the formation of two new C–C bonds that involve the both meta positions of the two 4-R-1,4-dihydropyridinate fragments, resulting the binuclear aluminium species [Me2Al(4-R-iPrHBIP)]2 (R = Bn (3a); allyl (3′a)). Experimental kinetics showed that the dimerization of 2′a obeys second order rate with negative activation entropy, which is consistent with a bimolecular rate-determining step. Controlled methanolysis of both 3a and 3′a release the metal-free dimeric bases, (4-Bn-iPrHBIPH)2 and (4-allyl-iPrHBIPH)2, providing a convenient route to these potentially useful ditopic ligands. When the R′ groups are bulkier than Me (2b, 2′b and 2′c), the dimerization is hindered or fully disabled, favoring the formation of paramagnetic NMR-silent species, which have been identified on the basis of a controlled methanolysis of the final organometallic products. Thus, when a toluene solution of [AlEt2(4-Bn-iPrBIPH)] (2b) was heated at 110 °C, followed by the addition of methanol in excess, it yields a mixture of the dimer (4-Bn-iPrHBIPH)2 and the aromatized base 4-Bn-iPrBIP, in ca. 1 : 2 ratio, indicating that the dimerization of 2b competes with its spontaneous dehydrogenation, yielding a paramagnetic complex containing a AlEt2 unit and a non-innocent (4-Bn-iPrBIP)˙− radical-anion ligand. Similar NMR monitoring experiments on the thermal behavior of [AlEt2(4-allyl-iPrBIPH)] (2′b) and [AliBu2(4-allyl-iPrBIPH)] (2′c) showed that these complexes do not dimerize, but afford exclusively NMR silent products. When such thermally treated samples were subjected to methanolysis, they resulted in mixtures of the alkylated 4-allyl-iPrBIP and non-alkylated iPrBIP ligand, suggesting that dehydrogenation and deallylation reactions take place competitively.
- This article is part of the themed collection: Inorganic chemistry of the p-block elements


 Please wait while we load your content...
Please wait while we load your content...
