Abstract
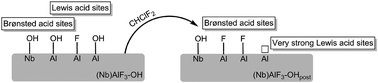
A niobium doped high surface aluminium fluoride (HS-AlF3) catalyst was prepared, using an approach in which niobium doped aluminium hydroxide fluoride obtained via reaction of aqueous HF with the respective metal alkoxides in isopropanol is further fluorinated under flow of CHClF2 at 200 °C. A comparable procedure was used to synthesize a Nb-free variant for comparison. Both catalysts exhibit very strong Lewis acidic surface sites which are capable to activate strong carbon–halogen bonds at room temperature, just as the classical high-surface AlF3 (HS-AlF3), obtained by reacting aluminium isopropoxide with anhydrous HF, does. The catalysts were characterized by elemental analysis, P-XRD, MAS NMR spectroscopy, N2 adsorption, NH3-TPD, and pyridine photoacoustic FT-IR spectroscopy. In contrast to previously reported niobium doped HS-AlF3, which was prepared using anhydrous HF, the doped catalyst obtained via this aqueous HF-route shows excellent performance both in the isomerization of 1,2-dibromohexafluoropropane, a reaction that occurs only in the presence of the strongest Lewis acids, and in the cyclization of citronellal to isopulegol, a reaction which requires both, Lewis and Brønsted acid sites.


 Please wait while we load your content...
Please wait while we load your content...