Transmetallation of bis(6-diphenylphosphinoxy-acenapth-5-yl)mercury with tin tetrachloride, antimony trichloride and bismuth trichloride†
Abstract
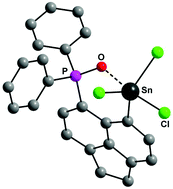
We recently communicated (Angew. Chem. Int. Ed., 2018, 57, 5917–5920) the transmetallation of [6-Ph2P(O)-Ace-5-]2Hg (1) with Pb(OAc)4, which, after work-up with HCl in Et2O, provided the first aryl lead trichloride, 6-Ph2P(O)-Ace-5-PbCl3 (2), and 6-Ph2P(O)-Ace-5-HgCl (3). With LiCl, 1 forms a complex, [{[6-Ph2P(O)-Ace-5]2Hg}2Li]Cl (1a), that shows no reactivity towards Pb(OAc)4 anymore, which highlights the role of the intramolecularly coordinated P(O) functionality in transmetallation. In this work, we have broadened the scope of the transmetallation reagent by the reaction of 1 with SnCl4, SbCl3 and BiCl3 giving rise to the formation of 6-Ph2P(O)-Ace-5-SnCl3 (4), 6-Ph2P(O)-Ace-5-SbCl2 (5) and 6-Ph2P(O)-Ace-5-BiCl2 (6), respectively. The high Lewis acidity permits 4 and 5 to undergo complexation with THF, whereas no such complexes were formed with 2 and 6. The molecular structures of 1a, 3, 4, 4·THF, 5, 5·THF and 6 were established by X-ray crystallography. The different P–O distances of 2, 4, 4·THF, 5, 5·THF and 6, reflecting the relative strength of the P(O)⋯E coordination (E = Pb, Sn, Sb, Bi), were discussed. The nature of the P(O)⋯E interactions of 2, 4, 4·THF, 5, 5·THF and 6 was studied using a set of real-space bond indicators (RSBIs).
- This article is part of the themed collection: Inorganic chemistry of the p-block elements


 Please wait while we load your content...
Please wait while we load your content...