Isolation of chloride- and hydride-bridged tri-iron and -zinc clusters in a tris(β-oxo-δ-diimine) cyclophane ligand†
Abstract
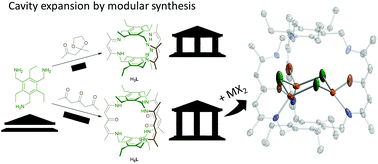
A cyclophane ligand (H6L) bearing three β-oxo-δ-diimine arms and the corresponding tri-iron and -zinc complexes in which the metal ions are bridged by either chlorides, viz. Fe3Cl3(H3L) (1) and Zn3Cl3(H3L) (2), or hydrides, viz. Fe3H3(H3L) (3), Zn3H3(H3L) (4), were synthesized and characterized. 1 adopts a chair-shaped C3v-symmetric [Fe3(μ-Cl)3]3+ cluster wherein only one hemisphere of the ligand is metallated and the other three ketoimine sites remain protonated as evidenced by single crystal X-ray diffraction and vibrational and NMR spectroscopic analyses. 3 and 4 were synthesized by substitution of the bridging chlorides in 1 and 2 using KBEt3H and are accessed with retention of the three protonated ketoimine sites.
- This article is part of the themed collection: The central role of the d-block metals in the periodic table


 Please wait while we load your content...
Please wait while we load your content...