C–F activation of perfluorophenazine at nickel: selectivity and mechanistic investigations†
Abstract
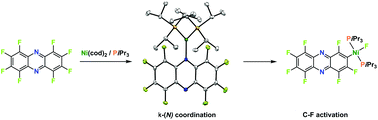
The reactivity of [Ni(cod)2] towards perfluorophenazine in the presence of phosphines is reported. When PiPr3 and PCy3 are used, an initial κ-(N) coordination of the nickel centre to the nitrogen atom of the perfluorophenazine ring occurs, forming the dark blue complexes [Ni{κ-(N)-C12N2F8}(PiPr3)2] (1) and [Ni{κ-(N)-C12N2F8}(PCy3)2] (2). Complex 1 was structurally characterized by X-ray diffraction analysis. The complexes rearranged by regioselective C–F activation of the perfluorophenazine ring in the 2-position to yield complexes trans-[NiF(2-C12N2F7)(PiPr3)2] (5) and trans-[NiF(2-C12N2F7)(PCy3)2] (6). The structure of 6 was also determined by X-ray diffraction analysis. Kinetic measurements for the decrease of 1 at different temperatures reveal a first order reaction with ΔH‡ = 19 ± 7 kcal mol−1. Initially, small amounts of an intermediate, assigned as [Ni(η2-1,2-C12N2F8)(PiPr3)2] (3), were observed, which exhibits a 1,2-η2 coordination of the perfluorophenazine. DFT calculations on the same transformation were also computed, which suggest that both a phosphine-assisted mechanism and an oxidative addition can be operating reaction pathways. The 1,2-η2 complex [Ni(η2-1,2-C12N2F8)(PEt3)2] (4) was obtained when PEt3 was used as ligand, and an unstable dark red complex trans-[NiF(2-C12N2F7)(PEt3)2] (7) formed rapidly by C–F activation. The reactivity of the perfluorophenazine was compared with those of perfluorodibenzo-p-dioxin. In this case, no prior coordination was observed and the C–F activation took place in a less selective manner forming trans-[NiF(1-C12O2F7)(PiPr3)2] (8) and trans-[NiF(2-C12O2F7)(PiPr3)2] (9), outlining the role of the nitrogen for the selectivity of the process. Treatment of two equivalents of [Ni(cod)2] and four equivalents of PiPr3 with perfluorophenazine afforded a double C–F activation to give [{trans-(PiPr3)2NiF}2(2,7-C12N2F6)] (10).


 Please wait while we load your content...
Please wait while we load your content...