Temperature-assisted formation of reversible metallophilic Au–Ag interaction arrays†
Abstract
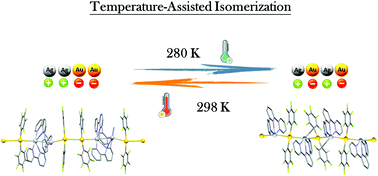
A temperature-controlled self-assembly process in a solution of [Ag(terpy)]nn+ and [Au(C6F5)2]− units has been performed. For this, the crystallisation of the complex [{Au(C6F5)2}Ag(terpy)]n under the same experimental conditions, changing only the temperature, allows the synthesis of polymorphs [{Au(C6F5)2}2Ag2(terpy)2]n (2a) at 298 K and [{Au(C6F5)2}Ag(terpy)]n (2b) at 280 K. The X-ray diffraction studies previously reported for 2a revealed a polymeric structure with an unusual + + − − + + − − charge sequence, whereas for polymorph 2b, a more classical + − + − disposition has been obtained. The conversion of one polymorph into the other can be achieved by simple dissolution of one of them and by recrystallisation at the corresponding temperature. The mechanism of the formation of each polymorph is proposed in view of their 1H NMR, 1H-PGSE NMR and molar conductivity measurements.


 Please wait while we load your content...
Please wait while we load your content...