A Mn(iv)–peroxo complex in the reactions with proton donors†
Abstract
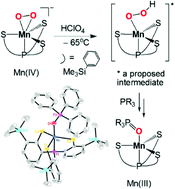
Protons play an important role in promoting O–O or M–O bond cleavage of metal–peroxo complexes. Treatment of side-on O2-bound [PPN][MnIV(TMSPS3)(O2)] (1, PPN = bis(triphenylphosphine)iminium and TMSPS3H3 = 2,2′,2′′-trimercapto-3,3′,3′′-tris(trimethylsilyl)triphenylphosphine) with perchloric acid (HClO4) in the presence of PR3 (R = phenyl or p-tolyl) results in the formation of neutral five-coordinate MnIII(OPR3)(TMSPS3) complexes (R = phenyl, 2a; p-tolyl, 2b), which are confirmed by X-ray crystallography. Isotope labelling experiments demonstrate that the oxygen atom in the phosphine oxide product derives from the peroxo ligand of 1. Reactions of 1 with weak proton donors, such as phenylthiol, phenol, substituted phenol and methanol, are also investigated to explore the reactivity of the MnIV–peroxo complex, leading to the isolation of a series of five-coordinate [MnIII(L)(TMSPS3)]− complexes (L = phenylthiolate, phenolate or methoxide). Mechanistic aspects of the reactions of the MnIV–peroxo complex with proton donors are discussed as well.


 Please wait while we load your content...
Please wait while we load your content...