Bipyridine-based iridium(iii) triplet emitters for organic light-emitting diodes (OLEDs): application and impact of phenyl substitution at the 5′-position of the N-coordinating pyridine ring†
Abstract
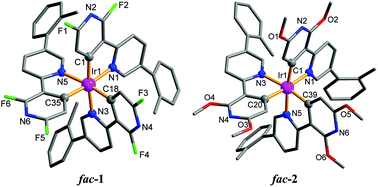
Three blue phosphorescent homoleptic iridium(III) complexes based on a bipyridine ligand were synthesized. The structures of these Ir(C^N)3 analogues were determined by single-crystal X-ray diffraction analysis. Two geometrical isomers, facial and meridional, formed as the major products, and the ratio of the products depended on the substituents. The photophysical and electrochemical properties of the complexes were analyzed, and they were used as dopants for the fabrication of phosphorescent organic light-emitting diodes (PHOLEDs). The dependence of current density on dopant concentration in the devices, as well as their external quantum efficiencies and current efficiencies, were evaluated. All complexes exhibited intense, sky-blue phosphorescent emission at λmax = 479, 484 and 488 nm, and the absolute quantum efficiencies in the thin films were high at 0.72, 0.75 and 0.81. A maximum current efficiency of 39.8 cd A−1 and an external quantum efficiency (EQE) of 14.9% were obtained, which signified superior performance among blue phosphorescent organic light-emitting diodes. High efficiencies of 39.2 cd A−1 and 14.0% EQE were still achieved at a luminance of 1000 cd m−2.
- This article is part of the themed collection: The central role of the d-block metals in the periodic table


 Please wait while we load your content...
Please wait while we load your content...