Polynuclear alkoxy–zinc complexes of bowl-shaped macrocycles and their use in the copolymerisation of cyclohexene oxide and CO2†
Abstract
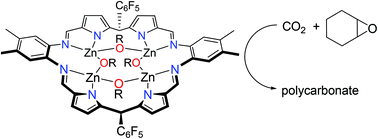
The reactions between alcohols and the tetranuclear ethyl–Zn complexes of an ortho-phenylene-bridged polypyrrole macrocycle, Zn4Et4(L1) 1 and the related anthracenyl-bridged macrocyclic complex, Zn4Et4(THF)4(L2) 2 have been studied. With long-chain alcohols such as n-hexanol, the clean formation of the tetranuclear hexoxide complex Zn4(OC6H13)4(L1) 3 occurs. In contrast, the use of shorter-chain alcohols such as i-propanol results in the trinuclear complex Zn3(μ2-OiPr)2(μ3-OiPr)(HL1) 4 that arises from demetalation; this complex was characterised by X-ray crystallography. The clean formation of these polynuclear zinc clusters allowed a study of their use as catalysts in the ring-opening copolymerisation (ROCOP) reaction between cyclohexene oxide and CO2. In situ reactions involving the pre-catalyst 1 and n-hexanol formed the desired polymer with the best selectivity for polycarbonate (90%) at 30 atm CO2, whilst the activity and performance of pre-catalyst 2 was poor in comparison.
- This article is part of the themed collection: Challenges in organometallic & coordination chemistry: in celebration of Geoff Cloke’s 65th birthday


 Please wait while we load your content...
Please wait while we load your content...