Oxidation reactions of a nucleophilic palladium carbene: mono and bi-radical carbenes†
Abstract
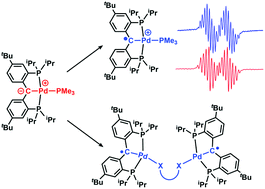
Palladium(II) cationic carbene radical and neutral bi-radical complexes were synthesized from a previously reported Pd(II) carbene in the presence of one and two electron oxidants. When [{PC(sp2)P}tBuPd(PMe3)] (1, [PC(sp2)P]tBu = (bis[2-(di-iso-propylphosphino)-4-tertbutylphenyl]methylene)) was treated with [Cp2Fe][X] (X = BArF4, ArF = 3,5-(CF3)2C6H3, or PF6), the corresponding radical cationic complexes [{PC˙(sp2)P}tBuPd(PMe3)][X] (2: X = BArF4, 3: PF6) were isolated and characterized. Magnetic moment measurements and EPR spectroscopy indicated the presence of a ligand centered unpaired electron. In the presence of two electron oxidants such as 1,8-naphthylene disulfide or 9,10-anthracenedione, 1 converts to [{PC˙(sp2)P}tBuPdS(C10H6)SPd{PC˙(sp2)P}tBu] (4) or [{PC˙(sp2)P}tBuPdO(C14H10)OPd{PC˙(sp2)P}tBu] (5), respectively. Single crystal X-ray diffraction and EPR spectroscopy confirmed the bi-radical nature of 4 and 5.
- This article is part of the themed collection: The central role of the d-block metals in the periodic table


 Please wait while we load your content...
Please wait while we load your content...
