Reactivity patterns for the activation of CO2 and CS2 with alumoxane and aluminum hydrides†
Abstract
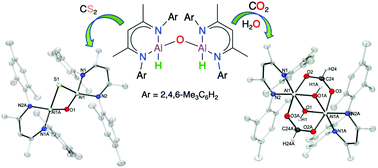
Carbon dioxide is readily fixed when reacting with either alumoxane dihydride [{MeLAl(H)}2(μ-O)] (1) or aluminum dihydride [MeLAlH2] (2) (MeL = HC[(CMe)N(2,4,6-Me3C6H2)]2–) to produce bimetallic aluminum formates [(MeLAl)2(μ-OCHO)2(μ-O)] (3) and [(MeLAl)2(μ-OCHO)2(μ-H)2] (5), respectively. Furthermore, [(MeLAl)2(μ-OCHO)2(μ-OH)2] (4) is easily obtained upon the reaction of 3 or 5 with H2O. The stability of the unusual dialuminum diformate dihydride core observed in 5 stems from the proximity of the Al centers allowing the formation of two Al–H⋯Al bridges and precluding further hydride transfer to the HCO2 moieties. Contrary to this behavior, 1 and 2 react with CS2 giving cyclic alumoxane and aluminum sulfides [(MeLAl)2(μ-S)(μ-O)] (6) and [{MeLAl(μ-S)}2] (7), respectively. The molecular structures of 3–7 were characterized by IR, Raman, solution or solid-state (MAS) NMR spectroscopy and mass spectrometry and for 4–7 were characterized by X-ray diffraction studies. NMR kinetic studies and DFT calculations suggest that the mechanisms for the formation of 6 and 7 involve the transfer of a hydride group forming transient aluminum thioformate intermediates which proceed to form Al–S–Al moieties through the cleavage of C–S bonds and insertion of a sulfur atom, followed by the elimination of thioformaldehyde.
- This article is part of the themed collections: Celebrating recent chemical science in Mexico and Inorganic chemistry of the p-block elements


 Please wait while we load your content...
Please wait while we load your content...