A phenalenyl-based nickel catalyst for the hydroboration of olefins under ambient conditions†
Abstract
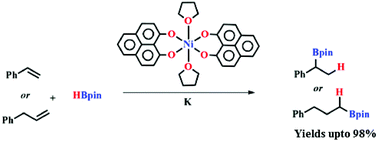
In this report, nickel-catalyzed hydroboration of vinylarenes and aliphatic alkenes is investigated. The non-innocent phenalenyl ligand moiety in the nickel complex Ni(PLY)2(THF)2 (1) was utilized as an electron reservoir for the selective hydroboration reaction in the presence of pinacolborane under ambient conditions. The mechanistic investigations revealed that the alkene hydroboration reaction takes place through a single electron transfer (SET) from the phenalenyl ligand backbone leading to the cleavage of the B–H bond.
- This article is part of the themed collection: Inorganic chemistry of the p-block elements


 Please wait while we load your content...
Please wait while we load your content...