P-Chiral 1,7-diphosphanorbornenes: from asymmetric phospha-Diels–Alder reactions towards applications in asymmetric catalysis†
Abstract
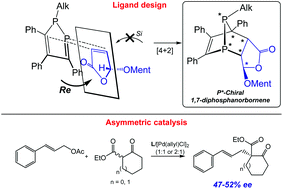
A straightforward synthesis of P-chiral polycyclic phosphines by an asymmetric Diels–Alder reaction of 1-alkyl-1,2-diphospholes and (5R)-(L-menthyloxy)-2(5H)-furanone (MOxF) is presented. The [4 + 2] cycloaddition reaction of 1,2-diphospholes 1–3 with MOxF (4) proceeded with high diastereoselectivity (de up to 90%) resulting in the corresponding enantiopure anti-endo-1,7-diphosphanorbornenes 5a–7a. The absolute configuration of 5–7 was proved by a variety of 1D/2D NMR correlation methods. The use of the anti-endo-1,7-diphosphanorbornene 5a in the Pd-catalyzed asymmetric allylic alkylation of cinnamyl acetate 8 with cyclic β-ketoesters 9a,b provided up to 52% ee.


 Please wait while we load your content...
Please wait while we load your content...