A molecular noble metal-free system for efficient visible light-driven reduction of CO2 to CO†
Abstract
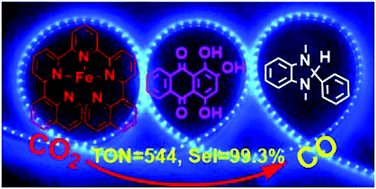
A new pentadentate quinoline–pyridine ligand and its iron (1), cobalt (2) and nickel (3) complexes have been synthesized and characterized. The iron complex exhibits excellent photocatalytic activity towards CO2-to-CO conversion with a TON(CO) of 544 and a selectivity of 99.3% using the commercially available organic dye purpurin as the photosensitiser and BIH as the electron donor. In contrast, the cobalt and nickel complexes result in very low activity for CO production with a TON of only 8 and 15, respectively. On the other hand, all the three complexes show good electrocatalytic activity for CO2 reduction when using 2,2,2-trifluoroethanol as the proton source with the active intermediate of M0 species. The lack of activity in photocatalytic CO2 reduction by 2 and 3 can be attributed to the redox potential of MI/M0 which is significantly more negative than that of PP−/PP2− while in the case of 1 the FeI/Fe0 redox potential becomes more positive than that of the PP−/PP2− couple.
- This article is part of the themed collection: The central role of the d-block metals in the periodic table


 Please wait while we load your content...
Please wait while we load your content...