Conjugated, rigidified bibenzimidazole ancillary ligands for enhanced photoluminescence quantum yields of orange/red-emitting iridium(iii) complexes†
Abstract
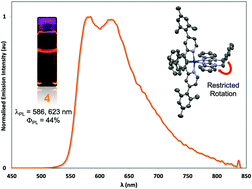
A series of six novel [Ir(C^N)2(N^N)](PF6) complexes (C^N is one of two cyclometalating ligands: 2-phenyl-4-(2,4,6-trimethylphenyl)pyridine, MesppyH, or 2-(napthalen-1-yl)-4-(2,4,6-trimethylphenyl)pyridine, MesnpyH; N^N denotes one of four neutral diamine ligands: 4,4′-di-tert-butyl-2,2′-bipyridine, dtbubpy, 1H,1′H-2,2′-bibenzimiazole, H2bibenz, 1,1′-(α,α′-o-xylylene)-2,2′-bibenzimidazole, o-xylbibenz or 2,2′-biquinoline, biq) were synthesised and their structural, electrochemical and photophysical properties comprehensively characterised. The more conjugated MesnpyH ligands confer a red-shift in the emission compared to MesppyH but maintain high photoluminescence quantum yields due to the steric bulk of the mesityl groups. The H2bibenz and o-xylbibenz ligands are shown to be electronically indistinct to dtbubpy but give complexes with higher quantum yields than analogous complexes bearing dtbubpy. In particular, the rigidity of the o-xylbibenz ligand, combined with the steric bulk of the MesnpyH C^N ligands, gives a red-emitting complex 4 (λPL = 586, 623 nm) with a very high photoluminescence quantum yield (ΦPL = 44%) for an emitter in that region of the visible spectrum. These results suggest that employing these ligands is a viable strategy for designing more efficient orange-red emitters for use in a variety of photophysical applications.
- This article is part of the themed collection: The central role of the d-block metals in the periodic table


 Please wait while we load your content...
Please wait while we load your content...