Substituent dependence on the spin crossover behaviour of mononuclear Fe(ii) complexes with asymmetric tridentate ligands†
Abstract
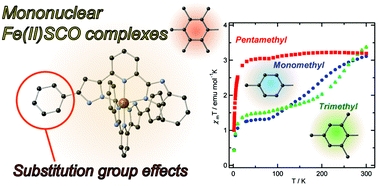
Three mononuclear iron(II) complexes of the formula [FeII(H2L1–3)2](BF4)2·x(solv.) (H2L1–3 = 2-[5-(R-phenyl)-1H-pyrazole-3-yl] 6-benzimidazole pyridine; H2L1: R = 4-methylphenyl, H2L2, R = 2,4,6-trimethylphenyl, H2L3, R = 2,3,4,5,6-pentamethylphenyl) (1, H2L1; 2, H2L2; 3, H2L3) with asymmetric tridentate ligands (H2L1–3) were synthesized and their structures and magnetic behaviour investigated. Significant structural distortions of the dihedral angles between phenyl and pyrazole groups were observed and found to depend on the nature of the substituent groups. Cryomagnetic studies reveal that 1 and 2 show gradual spin crossover behavior, while 3 remains in the high spin state between 1.8 and 300 K.


 Please wait while we load your content...
Please wait while we load your content...