New Pt→M (M = Ag or Tl) complexes based on anionic cyclometalated Pt(ii) complexes†
Abstract
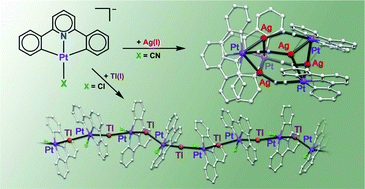
Anionic cyclometalated complexes (NBu4)[Pt(CNC)X] (X = Cl (1), CN (2), or S-2py (pyridine-2-thiolate) (3); –CNC– = 2,6-di(phen-2-ide)-pyridine) have been used as precursors in the synthesis of heteropolynuclear Pt–Ag or Pt–Tl complexes containing donor–acceptor metal–metal bonds. Their reaction with AgClO4 or [Ag(OClO3)(PPh3)] produces complexes in which the nuclearity and structure seem to be determined by the ability of the ligand X to form bridges between the metals. Thus, the characteristic linear bridging of the cyano ligand leads to the formation of an octanuclear [{Pt(CNC)(μ-CN)}4Ag4] (4) or tetranuclear [{Pt(CNC)(μ-CN)}2{Ag(PPh3)}2] (6) complex, with CN bridges between different “Pt–Ag” units. However, the S-2py ligand can act as a bridge between Pt and Ag of the same “Pt–Ag” unit, giving rise to the complex [{Pt(CNC)(S-2py)}2Ag2]·CH2Cl2 (5). On the other hand, the reaction of 1–3 with TlPF6 yields the complexes [PtTl(CNC)Cl] (7), [PtTl(CNC)(CN)] (8), and (NBu4)[{Pt(CNC)(S-2py)}2Tl] (9), while with [Tl(S-2py)], [PtTl(CNC)(S-2py)] (10) is obtained. The structures of all these Pt–Tl complexes show great variation, with several geometric arrangements which sometime co-exist in the same crystal structure: discrete Pt–Tl, Pt–Tl–Pt and Pt–Tl–Pt–Tl units, as well as infinite ⋯Pt–Tl–Pt–Tl⋯ chains. This variability could be due to the lability of the Pt–Tl bonds and the ability of the thallium center to establish secondary interactions with donor atoms or aromatic π electron density from neighboring moieties.


 Please wait while we load your content...
Please wait while we load your content...