Carboxyethyltin and transition metal co-functionalized tungstoantimonates composited with polypyrrole for enhanced electrocatalytic methanol oxidation†
Abstract
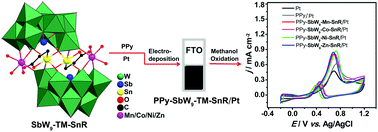
Carboxyethyltin and first-row transition metals (TMs) were firstly introduced into trivacant Keggin-type tungstoantimonate in an aqueous solution, leading to the formation of four crystalline organic–inorganic hybrid sandwich-type polyoxometalates (POMs), formulated as Na10−x−yKyHx[((TM)(H2O)3)2(Sn(CH2)2COO)2(SbW9O33)2]·nH2O (SbW9-TM-SnR, TM = Mn, Co, Ni, Zn; x = 1, 1, 0, 0; y = 0, 5, 5, 2; n = 18, 24, 24, 22, respectively). SbW9-TM-SnR exhibit high catalytic ability for the oxidation of cyclohexanol. Meanwhile, SbW9-TM-SnR were composited with polypyrrole (PPy) through an electropolymerization process, forming PPy-SbW9-TM-SnR, on which platinum (Pt) was further electro-deposited to prepare PPy-SbW9-TM-SnR/Pt for electrocatalytic methanol (CH3OH) oxidation in acid solution. The composition and morphology of PPy-SbW9-TM-SnR/Pt were determined by IR, scanning electron microscopy (SEM), energy dispersive spectroscopy (EDS) and X-ray photoelectron spectroscopy (XPS). The electrochemical experimental results show that SbW9-TM-SnR and PPy obviously enhance the electrocatalytic and anti-intoxication abilities of Pt, and the highest peak current density of 0.87 mA cm−2, corresponding to 1.85 and 1.43 times higher than those of pure Pt and PPy/Pt electrodes respectively, is acquired for the PPy-SbW9-Ni-SnR/Pt composite electrode. These findings may enlarge the application of PPy and POMs in the electrocatalytic field.


 Please wait while we load your content...
Please wait while we load your content...