Synthesis, structure and palladium coordination of ambiphilic, pyridine- and phosphine-tethered N-boryl imine ligands†
Abstract
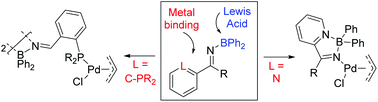
We describe here the synthesis and structural characterization of two new classes of ambiphilic, N-boryl imine ligands, wherein boron is associated with a Lewis basic imine nitrogen. These ligands can be easily generated in two steps from the corresponding pyridinyl- and phosphinyl-tethered aldehydes. 11B NMR analysis suggests the association of the Lewis acidic boron to either the pyridine unit or via intermolecular acid/base interactions with the imine. Both of these ligands can coordinate to palladium, and their structures were confirmed by X-ray crystallography.


 Please wait while we load your content...
Please wait while we load your content...