Selective reduction of formamides to O-silylated hemiaminals or methylamines with HSiMe2Ph catalyzed by iridium complexes†
Abstract
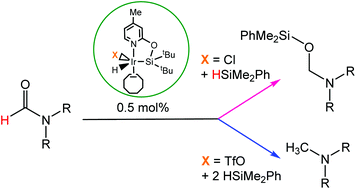
The reaction of (4-methyl-pyridin-2-iloxy)ditertbutylsilane (NSitBu–H, 1) with [IrCl(coe)2]2 affords the iridium(III) complex [Ir(H)(Cl)(κ2-NSitBu)(coe)] (2), which has been fully characterized including X-ray diffraction studies. The reaction of 2 with AgCF3SO3 leads to the formation of species [Ir(H)(CF3SO3)(κ2-NSitBu)(coe)] (3). The iridium complexes 2 and 3 are effective catalysts for the reduction of formamides with HSiMe2Ph. The selectivity of the reduction process depends on the catalyst. Thus, by using complex 2, with a chloride ancillary ligand, it has been possible to selectively obtain the corresponding O-silylated hemiaminal by reaction of formamides with one equivalent of HSiMe2Ph, while complex 3, with a triflate ligand instead of chloride, catalyzed the selective reduction of formamides to the corresponding methylamine.
- This article is part of the themed collection: Inorganic chemistry of the p-block elements


 Please wait while we load your content...
Please wait while we load your content...