One-pot cascade syntheses of microporous and mesoporous pyrazine-linked covalent organic frameworks as Lewis-acid catalysts†
Abstract
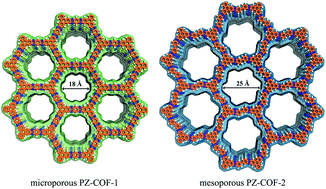
Covalent organic frameworks (COFs) are crystalline porous solids with broad potential applications. So far, the successful construction of COFs has been limited to a few condensation reactions, and nearly all COFs were obtained by single-step synthesis based on predesigned linkers. Here, we report a general strategy in view of a one-pot cascade reaction to prepare both microporous and mesoporous fully π-conjugated pyrazine-linked COF materials (PZ-COFs). The obtained PZ-COFs show high chemical stability, large specific surface areas and promising H2, CH4 and CO2 uptake capacities. Furthermore, we demonstrate that manganese(II)-incorporated PZ-COFs can act as excellent Lewis-acid catalysts for the cyanosilylation of aromatic aldehydes. This study not only provides a facile method to synthesize COFs required for multistep reactions but also expands the applications of COFs as promising catalysts.
- This article is part of the themed collection: New Talent: Asia-Pacific


 Please wait while we load your content...
Please wait while we load your content...