Redox chemistry of an anionic dithiolene radical†
Abstract
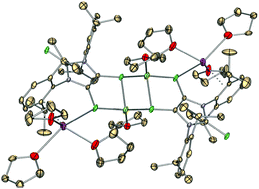
The redox chemistry of the first stable anionic dithiolene radical 1˙ was investigated by both reactivity and cyclic voltammetry studies. While one-electron reduction of 1˙ by Cp2Co or KC8 affords the corresponding dithiolate dimers 2 and 3, respectively, one-electron oxidation of 1˙ by Ph3C+BF4− (or O2) conveniently gives 4, the neutral dithiolene dimer.
- This article is part of the themed collection: Inorganic chemistry of the p-block elements


 Please wait while we load your content...
Please wait while we load your content...
